-
Products
- Lab Instruments
-
Lab Meters and Probes
Calibration Standards Sension+ Meters and ProbesOther Meters and Probes Radiometer Probes
- Chemistries, Reagents, and Standards
-
Online Analyzers
Ammonium Analysers Ammonia Monochloramine Analyzers Chlorine Analyzers
- CL17sc
- CL10sc Amperometric
- 9184 sc Amperometric
- Ultra Low Range CL17sc Colorimetric Chlorine Analyser
EZ Series Analysers- Iron
- Aluminium
- Manganese
- Phosphate
- Chloride
- Cyanide
- Fluoride
- Sulphate
- Sulphide
- Arsenic
- Chromium
- Copper
- Nickel
- Zinc
- Ammonium
- Total Nitrogen
- Total Phosphorus
- Phenol
- Volatile Fatty Acids
- Alkalinity
- ATP
- Hardness
- Toxicity
- Sample Preconditioning
- Boron
- Colour
- Nitrate
- Nitrite
- Silica
- Hydrogen Peroxide
- EZ Series Reagents
- EZ Series Accessories
- EZ sc Series Inorganics
- EZ sc Series Metals
- EZ sc Series Nutrients
- Flow and Collections
-
Online Sensors and Controllers
pH & ORP Sensors
- 12mm pH/ORP
- 8362 sc High Purity
- Combination pH/ORP
- Differential pH
- Digital Differential ORP
- Digital Differential pH
- LCP ORP
- LCP pH
Conductivity Sensors- 3400 Analogue Contacting
- 3400 Digital Contacting
- 3700 Analogue Inductive
- 3700 Digital Inductive
- 9523 Cation Conductivity
- 9525 DCCP System
- Drinking Water Panels
- Dual Parameter Monitoring Panel
- Single Parameter Monitoring Panels
- Water Quality Monitoring Panel (WQMP)
- Automated Lab Systems
-
Multiparameter Online Panels
Single, Dual, Multi-parameter Online Panels
- Drinking Water Panels
- Dual Parameter Monitoring Panel
- Single Parameter Monitoring Panels
- Water Quality Monitoring Panel (WQMP)
- Claros Water Intelligence System
- Test Kits & Strips
-
Microbiology
Prepared Media
- BARTS
- Liquid MPN
- MUG Tube
- Membrane Filtration
- Paddle Testers
- Presence-Absence
- Total Count Media
- Yeast and Mold
Labware- Accessories
- Funnels, Pumps & Manifolds
- Microbiology Filters
- Petri Dishes & Accessories
- Sampling Bags
- Vials, Tubes, Bottles & Racks
-
Lab Equipment and Supply
ApparatusInstrumentsGeneral Lab Consumables Safety EquipmentBooks and Reference Material Glassware/Plasticware
- Samplers
-
Hach eLearning
Lab Product Training Process Product Training
- AN-ISE sc
- Amtax sc
- B3500
- B7000
- CL17sc
- Differential pH Sensor
- Filtrax Sample Filtration Systems
- LDO sc
- Nitratax sc
- Orbisphere 6110
- Phosphax sc
- SC1000
- SC200
- SC4500
- Solitax sc
- TU5300sc/TU5400sc
- Electrochemistry
- Parameters
- Software Solutions
- Industries
- Support
- News & Events
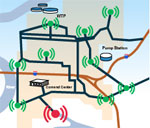
Application - Distribution Monitoring
Application Overview ??

Monitoring specific parameters in the distribution system is commonly a regulatory requirement to assure delivery of safe drinking water to the community. Additional monitoring can also provide early indication of nitrification issues, water infiltration, line breaks, extended water age, or potential security violations.
Feature ??
Homeland Security Technologies
Water quality and security from source to tap
At Hach Homeland Security Technologies, we provide complete solutions for monitoring drinking water quality and security from the raw water intake of the drinking water plant to the end-user after miles of traveling through the distribution system. Hach Homeland Security Technologies develops breakthrough technologies that put you in command of your entire water distribution and source water monitoring systems.
System Maintenance ??
Featured Products ??
Water Distribution Monitoring Panel sc
Product #: 6846000
The WDMP sc is a multiparameter on-line panel that assists in identifying potential problems that pose risks to public health when they occur in a distribution system. The panel consists of instrumentation for chlorine, turbidity, pH, conductivity, pressure and temperature. Conductivity and pH probes are held in a sensor manifold and can also be used for ORP, dissolved oxygen or other parameters as they become available.



 Recurring Orders
Recurring Orders